Science Blog: Separation of uranium from mine tailings water using a recyclable bioactive polymer
A sustainable approach to separate uranium from mine process effluents was proposed under the project “Advanced technologies for sustainable exploitation of uranium-bearing mineral resources” funded by the Academy of Finland (2015–2019), and led by the University of Eastern Finland in cooperation with the Geological Survey of Finland and the University of Tampere. A bioactive polymer that is derived from renewable sources was utilised as a low-cost adsorptive material to treat tailings water and recover uranium.
There are various treatment strategies for removing uranium from liquid effluents, including chemical precipitation, solvent extraction and membrane separation, ion exchange and adsorption, electrocoagulation, and biological and passive treatment methods. All the techniques have their own limitations; however, when looking for a selective and cost-efficient separation and recovery method for metals from side streams and effluents, adsorption can be considered as one of the most promising technologies.
Hence, our study was directed towards an application of adsorption as a treatment method and evaluation of the effectiveness of uranium separation from tailings water using chitosan, a recyclable natural biopolymer. Chitosan is a material with a high affinity and adsorption capacity for various metal ions. It can preferentially recover uranium over other metals from industrial wastewaters with complex chemical compositions and can easily be regenerated and reused during the treatment process. The mine process effluents used in this study originated from the mineral processing tests on a uranium-bearing ore sample carried out by Dr Antti Taskinen at the GTK Mineral Processing Laboratory in Outokumpu.
The conducted research aimed to improve understanding of the simultaneously occurring removal mechanisms and factors limiting uranium separation from a multi-component system. To assess the potential of the proposed treatment solution, various operational process parameters were examined (pH, temperature, adsorbent dose) and optimised. The solid phase characterisation of the applied biopolymer included pHZPC, FTIR-ATR and SEM-EDS (Figure 1). Comprehensive information on the experimental approach and a thorough discussion on the kinetics of uranium uptake at various material doses, the adsorption equilibrium and the influence of temperature and pH on the treatment performance of uranium-bearing effluents is presented in our recent work (Szlachta et al. 2020).

We also investigated the chemical regeneration of spent biopolymer and its recyclability. Regenerability and recyclability are desirable features of adsorbents developed and produced for large-scale water and wastewater treatment applications. Extending the life cycle of the chitosan used for the treatment of industrial effluents is of high importance in evaluating the overall effectiveness and economic feasibility of the process. However, the saturated biopolymer becomes toxic and hardly biodegradable. Thus, before the final elimination of spent material from the system, it should be completely recovered (Vakili et al. 2019). If options for regeneration or recycling are limited, then the material should be stabilised prior to disposal.
Experiments were conducted using flotation tailings water (47.01 mg U/L and a pH of 3.3), and the results are illustrated in Figure 2. It was proven that sodium salt was ineffective, and the adsorbed uranium was only partly releasable. Under alkaline conditions, the removal of uranium was accompanied by the precipitation of iron present in flotation tailings water (the mass of the solids increased by 25.2%), and the uranium ions were enclosed in a matrix of precipitating iron oxides, making them unavailable for desorption with NaHCO3 (Noubactep et al. 2006). The tests demonstrated that acidic eluents were more suitable for desorbing uranium ions from the saturated biopolymer, but material decomposition and mass loss were observed. Sufficient biopolymer stability and a high recycling efficiency were obtained for less concentrated solutions of HCl.
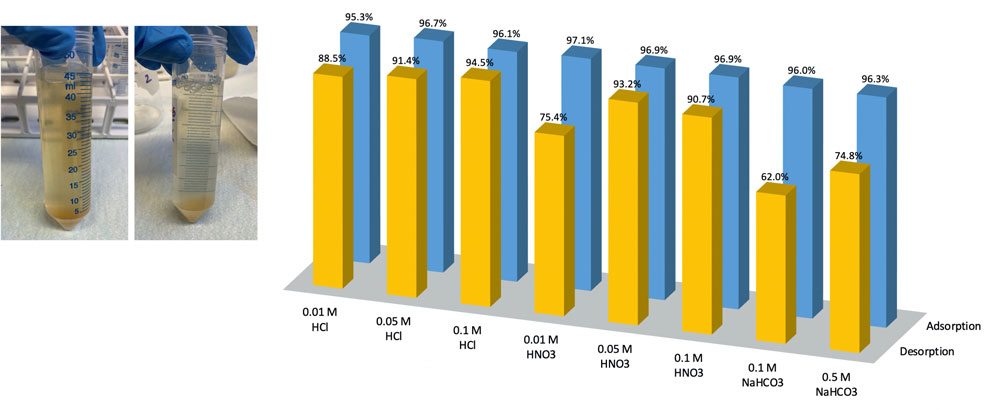
The reusability of the biopolymer is an essential factor influencing its practical applicability, but the specifics of this material limit its regeneration cycles. On the other hand, its low price makes it economically attractive, especially compared to commercially available ion exchange resins and synthetic adsorbents. Industrial-grade chitosan with various physicochemical properties is available from 10 USD/kg. The total operating costs include labour, maintenance, and process-specific costs, such as adsorbent and energy consumption costs. In this case, long-term savings on operating costs are guaranteed, as the source of chitosan (chitin) is inexpensive and renewable. However, to provide a rational estimation of the process-specific operating costs and compare the economic feasibility of the proposed approach with other alternative solutions, scaling-up of the process, from the laboratory to the pilot scale, is recommended.
References
Noubactep, C., Schöner, A. & Meinrath, G. 2006. Mechanism of uranium removal from the aqueous solution by elemental iron. Journal of Hazardous Materials 132, 202. Available at: doi.org/10.1016/j.jhazmat.2005.08.047
Szlachta, M., Neitola, R., Peräniemi, S. & Vepsäläinen, J. 2020. Effective separation of uranium from mine process effluents using chitosan as a recyclable natural adsorbent. Separation and Purification Technology, 253, 117493. Available at: doi.org/10.1016/j.seppur.2020.117493
Vakili, M. S., Cagnetta, D. G., Wang, W., Meng, P., Liu, D. & Yu, G. 2019. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Separation and Purification Technology 224, 373-387. Available at: doi.org/10.1016/j.seppur.2019.05.040
Text: Małgorzata Szlachta and Raisa Neitola
Dr Małgorzata Szlachta works as a senior scientist at the Geological Survey of Finland, in the Circular Economy Solutions Unit. She joined GTK in 2018. She is also affiliated with the Wrocław University of Science and Technology in Poland, where she holds a position as an Assistant Professor. She gained her postdoctoral experience at the Utrecht University in the Netherlands, and conducted short-term research stays at the University of Malaya in Malaysia and the ESRF in France. She has a PhD in the field of environmental engineering and specialises in water and wastewater treatment technologies. Her current focus areas are R&D solutions aimed at active methods for mine water treatment, water reuse and recycling, and the recovery of valuable elements.
Dr Raisa Neitola is a head of project management in the operative management team. She has been at the Geological Survey of Finland since 2009. Raisa Neitola has worked as a senior scientist at GTK Mintec in Outokumpu, carrying out jointly funded national and international R&D projects. Her research interests are focused on developing a sustainable beneficiation process for raw materials and the circular economy. She has specialised in process chemistry and hydrometallurgical technologies.
